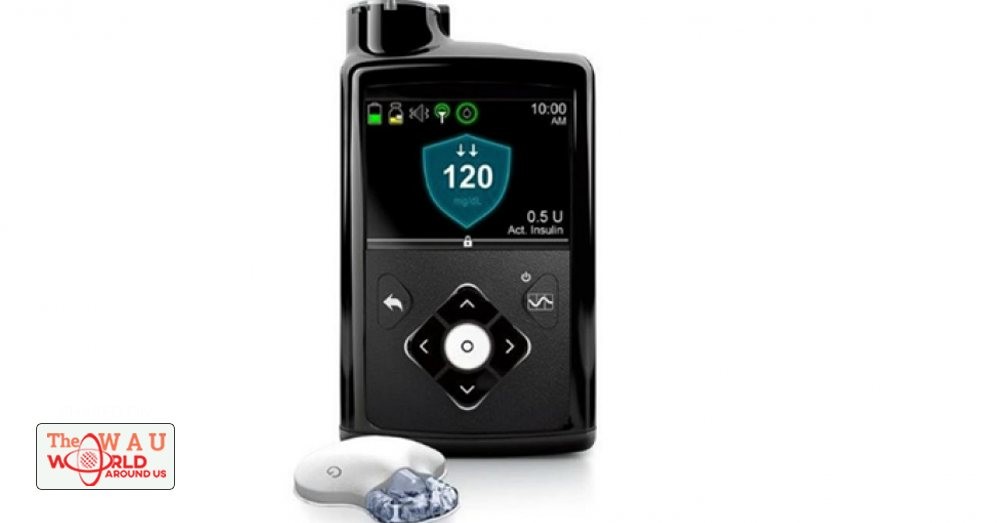
The very first “artificial pancreas” was approved by the Food and Drug Administration yesterday, STAT reports. The device, which developer Medtronic calls the MiniMed 670G, is an insulin pump that incorporates an algorithm that wirelessly corresponds with a person’s glucose monitor and adjusts insulin dosage based on its readings. The MiniMed 670G acts not unlike cruise control, suspending insulin to the bloodstream if glucose dips too low, though patients will still have to manually control their glucose levels before they eat. The diabetes community fears the device will come with a sky-high price tag when it becomes available in spring of 2017, but is hopeful the MiniMed 670G will lead to safer and more worry-free lives.
Share This Post