There has been a global surge in demand for drugs normally used against malaria to tackle the coronavirus, as governments urgently seek out treatments for the new disease.
Chloroquine, and a related derivative, hydroxychloroquine, have gained attention - despite the World Health Organization (WHO) saying there is no definitive evidence they work.
So what is the current evidence of their effectiveness as a treatment for the coronavirus, and who is using them?
What do we know about these drugs?
President Trump has frequently referred to the potential of hydroxychloroquine in White House briefings. At a recent press conference, he referred to it and said: "What do you have to lose? Take it."
In a video removed by Facebook for breaching its misinformation guidelines, Brazilian President Jair Bolsonaro claimed "hydroxychloroquine is working in all places".
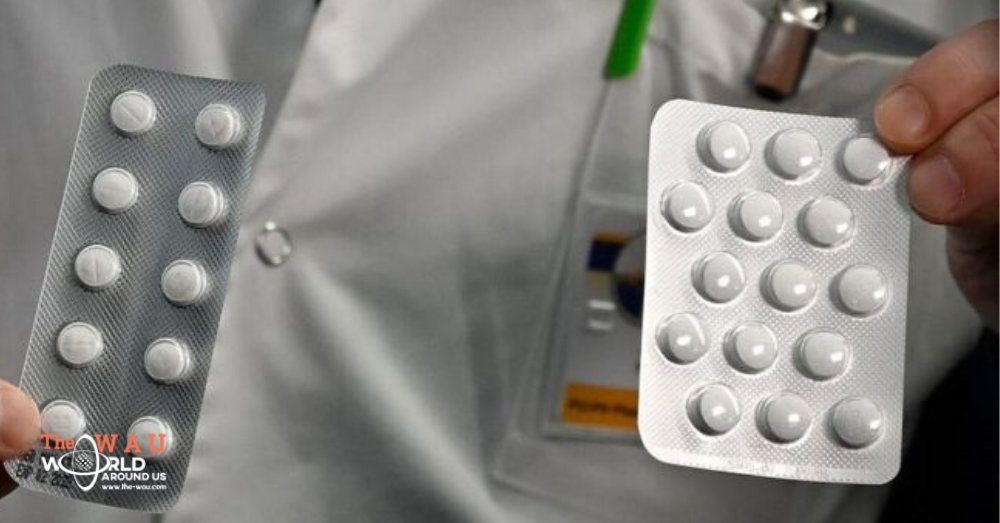
Tablets containing chloroquine have long been used in the treatment of malaria to reduce fever and inflammation, and the hope is that they can also inhibit the virus that causes Covid-19.
"Chloroquine seems to block the coronavirus in lab studies. There's some anecdotal evidence from doctors saying it has appeared to help," says James Gallagher, BBC health correspondent.
There is insufficient evidence at the moment from current trials as to their effective use in treatment of patients with Covid-19.
There are also risks of serious side effects, including renal and liver damage.
"We need larger, high-quality randomised clinical trials in order to better evaluate their effectiveness," says University of Oxford 's Kome Gbinigie, author of a report on anti-malarial testing for Covid-19.
More than 20 trials are being carried out, including in the US, UK, Spain and China.
Cabinet Office Minister Michael Gove says the UK is "conducting rapid clinical trials on anti-malarials" to assess if they are able to reduce the impact of Covid-19 on those affected.
In the US, various trials are under way for a combination of drugs including chloroquine, hydroxychloroquine and an antibiotic called azithromycin, for treating Covid-19 patients.
Which countries have authorised their use?
The US Food and Drugs Administration (FDA), the body in charge of licensing medicines in America, has granted "emergency use" authorisation for these drugs in the treatment of Covid-19 for a limited number of hospitalised cases.
That does not mean the FDA is saying they definitely work. But it does mean that in specific circumstances, hospitals can request and use the medicines from government stockpiles for use in Covid-19 treatment.
The US government has said that 30 million doses of hydroxychloroquine have been donated to the national stockpile by a German-based pharmaceutical company.
Other countries are also deploying these anti-malarial drugs to varying degrees.
France has authorised doctors to prescribe them for patients with Covid-19, but the country's medical watchdog has warned of side effects.
India's health ministry has recommended the use of hydroxychloroquine as a preventative treatment for healthcare workers, as well as households in contact with confirmed cases if they have a prescription from a doctor.
However, India's government research body has warned against the unrestricted usage of the anti-malarial drug and said it was "experimental" and only for emergency situations.
Several Middle Eastern countries have authorised its use or are conducting trials. This includes Bahrain (which claims it was one of the first countries to use hydroxychloroquine on coronavirus patients), Morocco, Algeria and Tunisia.
Is there enough chloroquine available?
As interest in these drugs has grown as a potential treatment for Covid-19, many countries have seen high demand and shortages.
Chloroquine and its derivatives have long been widely available in pharmacies, particularly in developing countries, for the treatment of malaria.
This is despite their declining efficacy against malaria, as the disease has become increasingly resistant.
Jordan has banned the sale of hydroxychloroquine in pharmacies to prevent stockpiling. Similarly, the Kuwaiti Health Ministry decided to withdraw all medicines containing the drugs from private pharmacies and limit them to hospitals and health centres.
Kenya has banned over-the-counter sales of chloroquine, so it is now only available on prescription.
India is a major producer of these antimalarial drugs, and has imposed a ban on exports.
President Trump has made a personal plea to India's Prime Minister, Narendra Modi, for access to the drugs for use in the United States. It has been reported that India is considering this request.
Unregulated use can be unsafe
In Nigeria, households still regularly use tablets containing chloroquine for treating malaria, even though it was banned in 2005 for first-line use because of its declining effectiveness.
News of a February study in China about the use of chloroquine for the coronavirus had already sparked lively debate in Lagos, so people began stocking up.
Following Mr Trump's reference to it as a coronavirus treatment, this ramped up, and shops and chemists sold out of the drug very quickly.
But the Nigerian Centres for Disease Control has told people to stop taking it. "The WHO has NOT approved the use of chloroquine for #COVID19 management."
Officials in Lagos state say there have been a number of people poisoned from overdoses of chloroquine.
Share This Post