Deliberately infecting healthy volunteers with the virus that causes Covid-19 may speed studies of vaccines against the deadly pathogen, the World Health Organization said.
Such studies, which pose significant potential dangers to subjects, may be considered in dire situations and with certain disclosures and protections, a working group of the United Nations health agency said in a report posted Wednesday on its website.
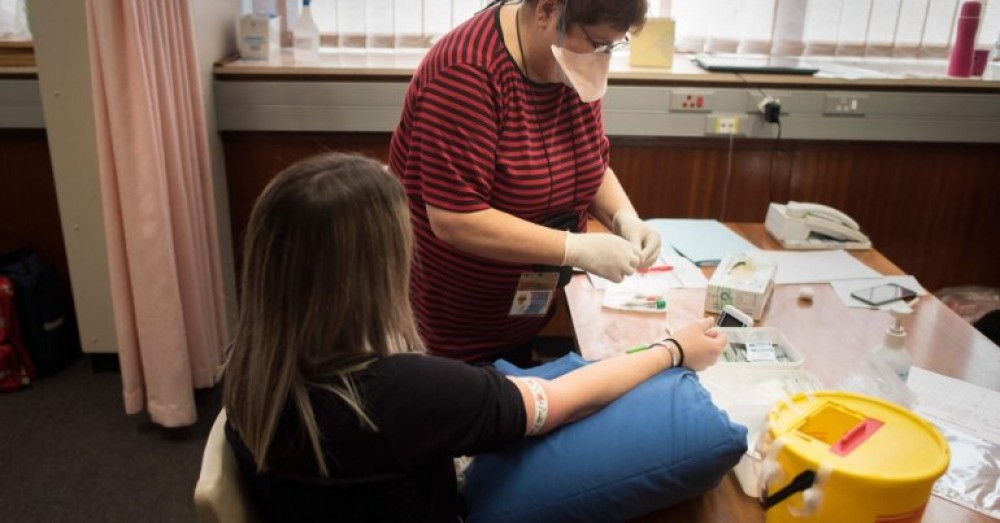
Researchers around the world are racing to develop vaccines to protect against the deadly coronavirus and allow countries to rebuild teetering economies. So-called challenge studies, where treatments or preventatives are tested directly against the infection in informed volunteers, might speed the path of vaccines to the public.
Challenge studies "can be substantially faster to conduct than vaccine field trials,” according to the working group paper, "in part because far fewer participants need to be exposed to experimental vaccines in order to provide (preliminary) estimates of efficacy and safety.”
The report laid out eight conditions that would need to be fulfilled for challenge studies to be considered, including scientific justification, an assessment of potential benefits and the fully informed consent of subjects.
Challenge studies hold the potential to reduce coronavirus mortality around the world but pose significant potential dangers for volunteers, scientists led by Marc Lipsitch, a Harvard School of Public Health epidemiologist, suggested in a recent paper.
"Obviously, challenging volunteers with this live virus risks inducing severe disease and possibly even death,” they said in March in an article in the Journal of Infectious Diseases.
Vaccines are usually tested in large groups, and the results are compared with a separate group of unvaccinated people. Waiting for both groups to become exposed to the illness in their regular daily lives can take months, while challenge studies would ensure that the subjects were promptly exposed to the virus.
Volunteers would typically be healthy, young adults at a relatively low risk of serious disease, and would be monitored and cared for, the scientists said.
Officials at Moderna Inc., where the lead vaccine for Covid-19 is in development, aren’t enthusiastic about the idea.
"I’m not sure I am a huge fan of it really for both practical and ethical reasons,” said Tal Zaks, Moderna’s chief medical officer, during a presentation put on by the health news organization Stat on Wednesday.
"As is oftenthe case, the devil is in the details.”
Zaks said he wasn’t sure that a challenge study, which could take months to design and implement, would speed development of the company’s product, which is already entering the second of three phases of study.
The shot would have to prevent the development of disease, not just mild infections, or it would be hard to know if the dose picked was the right one, he said.
Foundation 1daysooner has a website where people can sign up to participate in a human challenge trial for Covid-19, if one were to occur. Nearly 14,000 volunteers from 102 countries have signed up so far.
Share This Post